James McCormack PharmD, Aseem Malhotra MRCP and David Newman MD
National organisations such as NICE or American College of Cardiology (ACC)/American Heart Association (AHA) regularly release cardiovascular disease (CVD) guidelines that recommend lifestyle and medication interventions along with treatment thresholds based either on risk factors or calculated CVD risks. The release of these recommendations predictably leads to intense debate from healthcare organisations, government, industry, and clinicians on whether or not the recommendations are reasonable. At one extreme the recommendations are typically pilloried as being sufficiently weak to cost lives, while others insist the guidelines have threatened public health through over-medication.
This overwrought debate is rooted, we believe, in the mysterious imperative to provide treatment thresholds, rather than offer a reasonable synopsis of best evidence and its limitations so that patients and clinicians can apply individual values. Such a bestevidence
approach would support the increasing call – and need – for shared decision making in professional society guidelines. 1 Moreover, care guided by treatment thresholds largely
removes individual preference from the exam room while care explicitly informed by best evidence allows tailoring to a patient’s values and priorities.
When it comes to CVD risk factors, some population health advocates justify threshold-based guidelines by estimating how many heart attacks or strokes might be prevented across a population. This approach may be reasonable with vaccination for transmissible diseases, or public health regulations that demonstrate reliable and rapid real world reductions in morbidity and mortality (eg public smoking bans). However, risk factor modification with lifestyle interventions may for some individuals be unacceptably inconvenient, burdensome, or costly. In addition it is neither feasible nor desirable to speculate on a population’s values or preferences. Such questions are best left to individuals, as evidenced by one recent survey study demonstrating marked variation in negative values assigned to the act of taking daily pills: some respondents would refuse daily medications even if an inexpensive pill would cause no side-effects and add 10 years
to their life.2 What current guidelines therefore fail to address is the negative value assignments associated with commonly prescribed and recommended healthcare interventions.
Hopefully most clinicians and patients will agree with the principle that information is power. While not all patients will want to take the pills, undergo invasive procedures, or take on lifestyle changes commonly used for risk factor modification, all should have access to basic facts and evidence about the utility of these options.
Lifestyle interventions
Clinicians and patients should know that roughly 80 per cent of CVD can be attributed to modifiable lifestyle factors such as nutrition, physical activity and smoking. Patients should be counselled about the nature and value of a healthy diet – a Mediterranean
diet in moderation, with as little processed food as possible, is a cardiovascular intervention tested in randomised trials and shown to reduce CVD events.3 Patients should know that physical activities, particularly enjoyable ones, can lead to important, lasting
health and quality of life benefits.4 Finally, patients should be counseled and supported to quit smoking. While associated with some costs and inconvenience, these three interventions rarely include significant harm risk, can prolong life, and have the added
advantage of substantial non-cardiovascular benefits. Still, the decision to capitalise will remain individual.
Theoretical benefits of risk-factor modification
Let us assume a person’s lifetime risk of CVD is that of a male with two CVD risk factors, roughly 50 per cent.5 Now let us assume that with multiple risk-factor modification we can
reduce that risk relatively by 60 per cent, an optimistic assumption. This would shift a person’s lifetime risk for CVD from 50 per cent to 20 per cent. In this best-case scenario approximately 30 per cent of individuals benefit, but 70 per cent do not, even despite a lifetime of treatment. This is critical for clinicians and patients to appreciate: not everyone benefits when they reduce CVD risk factors.
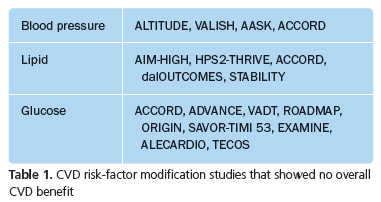
Indeed it may be equally important for patients and physicians to understand that over the last five or so years, a number of large, well designed studies have shown either no benefit or harm from interventions (primarily medications) that successfully modified CVD risk factors (see Table 1). Thus not only do a limited percentage of patients benefit, but in the case of risk factor modification with medications the endeavor may not always lead to
a change in important clinical outcomes.
A potential solution
Below is an example of using best evidence to construct a script for offering information patients can use. We offer statins as an example, as they have likely been studied more than any risk reduction medication.
Best available evidence
Dozens of relatively well-designed studies looking at the impact of statins have been published, including studies of men and women up to the age of approximately 80, mostly taking the drug for about five years.
Benefits
Typically, for relatively healthy people who have never had a heart attack or stroke, between 0.5 and 2 per cent of study subjects over a period of five years avoided having one of these problems by taking a statin. This is equivalent to roughly 1 in every 200 or up to 1 in every 50 people who took a statin. While debated, statins likely have either no effect on mortality or at best a <0.5 per cent impact. In other words less than 1 in every 200 people
who took a statin lived longer because of it.6,7
For persons who have heart disease or a history of stroke roughly 2–3 per cent (about 1 in every 40) who took a statin avoided a future heart attack or stroke, and about 1 per cent fewer people (1 in every 100) died.8
Finally, we might be able to make a more precise assessment of your chances of avoiding a heart attack, stroke, or death by using your age, gender, and other factors as part of a risk calculation.
Harms
Muscle aches seem to occur in approximately 5–10 per cent who take a statin but it is not always easy to know if these are truly caused by the statin. The patient leaflet by the drug company Pfizer says “common side-effects that may affect up to 1 in 10 patients include sore throat, nausea, digestive problems, muscle and joint pain”.9 If you do get side-effects we can safely stop the statin and typically they go away. While some people may have concerns stopping statins, even in patients with established heart disease the worst case scenario risk of earlier death from statin cessation for a couple of weeks is approximately 1 in 10,000.10 It’s instructive to note that in the largest cholesterol survey up to three quarters of new statin users discontinued the drug within one year, mostly because of side-effects.11
Abnormal liver blood tests occur in about 2 per cent ( or 1 in 50), though nobody knows if this is important. Severe muscle or kidney damage occurs rarely, in about 1 in 10,000. Statins also increase blood glucose levels which leads to about 1 per cent more people (1 in every 100 taking statins) being diagnosed with type 2 diabetes, which could have an important negative impact on your life,12 but would not increase your risk of heart problems.
Caveats
Industry sponsored studies (which most of the statin studies were) probably represent a best-case scenario. Typically study subjects are carefully selected partly because they are likely to see the most benefits and fewest harms. For example 36 per cent of screened patients were excluded from the Heart Protection Study before the actual trial even began which had the potential to screen out many people who may have suffered from adverse effects from simvastatin, including muscle symptoms. 13 Also, most statin studies have not gone beyond five years, so we don’t yet know the long-term effects.
Ultimately it’s your decision
The decision to take or not take a statin is yours – even experts seem to disagree.14,15 If you decide to take a statin I will gladly support your decision. If not, that’s fine too. I’m here for you either way.
Professor Chris Ham, chief executive of UK health think tank the King’s Fund recently said “many doctors aspire to excellence in diagnosing disease. Far fewer unfortunately aspire to the same standards of excellence in diagnosing what patients want”.16 He’s absolutely correct. If we want to truly improve quality of care for patients we need greater transparency and to begin crafting guidelines that bring doctors and patients together rather than move them apart. It’s time for guideline writers to include shared decision making tools for individual patients.
Two authors of this paper have developed free tools (without advertising or conflicts of interest) that provide clinicians with easier access to the benefits and harms of interventions. Professor Newman is part of a group of clinicians that have developed a framework and rating system to evaluate therapies based on their patient-important benefits and harms. This can be found at thennt.com. Professor McCormack has developed an interactive CVD risk calculator that provides clinicians and patients with specific CVD risk assessments along with an estimate of the benefit and harms of interventions. This can be found at cvdcalculator.com
References
1. Ubel PA. N Engl J Med 2015;372(26):2475–7.
2. Fontana M, et al. Circulation 2014;129:2539–46.
3. The NNT. Mediterranean diet for secondary prevention after heart
attack. http://bit.ly/1OVad8y.
4. Academy of Medical Royal Colleges. Exercise; the miracle cure. Feb
2015. http://bit.ly/1zRxgwe
5. Berry JD, et al. N Engl J Med 2012;366(4):321–9.
6. Abramson JD, et al. BMJ 2013;347:f6123
7. McCormack J, et al. BMC Med Res Methodol 2013;13:134.
8. The NNT. Statins given for 5 years for heart disease prevention (with
known heart disease). http://bit.ly/1HvuI6d.
9. Pfizer. Lipitor. Patient Information Leaflet. August 2013.
10. Malhotra A, et al. Prescriber 2015;26(1–2);6–7.
11. Statin Usage. Key findings and implications. http://bit.ly/TE4sFT
12. UCSF. Almost half of type 2 diabetes patients report acute and chronic
pain. August 2012. http://bit.ly/1OVbvjZ.
13. Heart Protection Study Collaborative Group. Lancet 2002;360:7–22.
14. Redberg RF, Katz MH. JAMA 2012;307(14):1491–2.
15. Blaha MJ, et al. JAMA 2012;307(14):1489–90.
16. Mulley A, et al. Patient preferences matter. Stop the silent misdiagnosis.
King’s Fund, 2012. http://bit.ly/1DzCnoR.
Declaration of interests
None to declare.
James McCormacK is professor at the Faculty of Pharmaceutical Sciences, UBC, Vancouver, Canada; Dr Malhotra is honorary consultant cardiologist, Frimley Park Hospital, Surrey, and consultant clinical associate to the Academy of Medical Royal Colleges; and David Newman is director of clinical research and associate professor of emergency medicine, Department of Emergency Medicine, Icahn School of Medicine at Mt Sinai, New York, USA